Knowledge Sharing
2023.08.11
Preimplantation Genetic Testing for Monogenic disorders (PGT-M): A brief introduction and case sharing
Preimplantation Genetic Testing for Monogenic disorders (PGT-M), formerly known as Preimplantation Genetic Diagnosis (PGD), is a genetic diagnostic test performed on embryos generated during in vitro fertilization (IVF) before their transfer.
Monogenic genetic diseases can be classified based on their inheritance types:
Monogenic genetic diseases account for approximately 18% of the reasons for childhood hospitalizations and about 20% of infant mortality cases. While these diseases are relatively rare, the overall prevalence of all monogenic genetic diseases in newborns worldwide is approximately 10 in every 1,000 newborns.
During the IVF process, when the embryos reach the blastocyst stage (D5/D6), technicians will perform a biopsy of the trophectoderm cells using micromanipulation equipment. Approximately 5 cells are extracted while avoiding the inner cell mass. These cells will undergo whole-genome amplification and then be tested using the personalized designed probes. The following are some of the PGT-M case studies conducted at our hospital.
In this case, the first child of the couple was diagnosed with Pompe Disease. After both spouses underwent carrier screening for genetic diseases, it was found that they were both carriers of Pompe Disease. As a result, they sought PGT-M testing for embryo screening. Based on the testing results during the procedure, they ended up with one affected embryo, two carrier embryos, and one unaffected embryo.
In this case, the couple had a significant family history of Spinocerebellar ataxia. Therefore, they sought IVF treatment and PGT-M testing at Lee Women's Hospital. The testing results during the procedure revealed two embryos without the mutated gene. After implanting one of these embryos, the patient became pregnant, and subsequent amniocentesis results were normal, leading to a successful delivery.
The patient had a family history of Hemophilia B, with the mother being a carrier and the brother being affected by Hemophilia B. After confirming that the patient is a carrier of Hemophilia B, they chose to undergo PGT-M testing. In the first attempt of IVF treatment, they obtained an unaffected embryo and implanted it. The patient successfully became pregnant, and the subsequent amniocentesis results were normal, resulting in a successful delivery.
Population suitable for PGT-M
- Individuals who have previously given birth to or have had a child with a genetic disorder.
- Individuals or families with a history of specific genetic diseases.
- Positive results from carrier screening for genetic diseases.
What are Monogenic Genetic Disorders?
Monogenic genetic diseases (or single gene disorders) are hereditary disorders caused by mutations or abnormalities in a single gene. They are often referred to as Mendelian disorders. These diseases follow a clear inheritance pattern and mostly adhere to Mendel's laws, where parents pass on genetic traits to their offspring. This type of inheritance makes monogenic genetic diseases relatively easy to track and predict.Monogenic genetic diseases can be classified based on their inheritance types:
- Autosomal Dominant Inheritance: When one of the parents carries a mutated gene, there is a chance for the offspring to be affected. The probability of having an affected child with the mutated gene is 50%.
- Autosomal Recessive Inheritance: Both parents need to carry the mutated gene for the offspring to be affected. The disease is expressed only when the individual inherits two mutated genes.
- X-Linked Inheritance: The mutated genes of these diseases are located on the X or Y sex chromosomes, and can be subdivided into: X-linked dominant inheritance, X-linked recessive inheritance, and Y-linked inheritance.
Monogenic genetic diseases account for approximately 18% of the reasons for childhood hospitalizations and about 20% of infant mortality cases. While these diseases are relatively rare, the overall prevalence of all monogenic genetic diseases in newborns worldwide is approximately 10 in every 1,000 newborns.
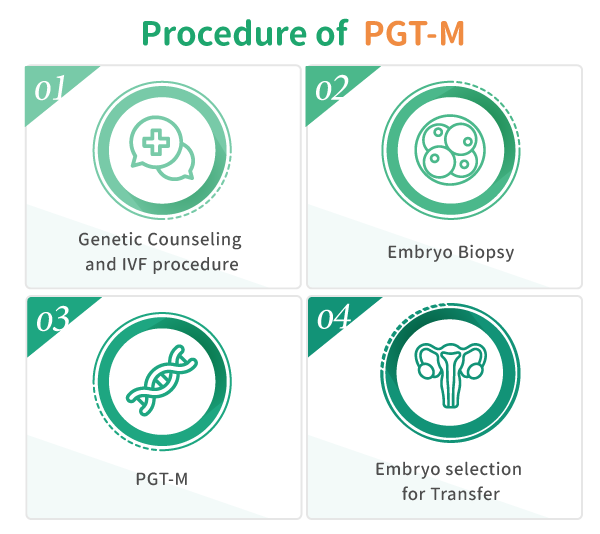
What is the procedure of IVF with PGT-M?

- Genetic Counseling (After confirming the genetic report, personalized probe design is carried out)
- Ovulation Induction
- Egg Retrieval and Sperm Collection
- In Vitro Fertilization and Embryo Culture
- Embryo Biopsy and PGT-M
- Embryo Transfer
How does PGT-M work?
Before undergoing PGT-M, patients must first undergo genetic counseling evaluation and confirm the genetic report. Then, the laboratory will conduct personalized probe design and testing.During the IVF process, when the embryos reach the blastocyst stage (D5/D6), technicians will perform a biopsy of the trophectoderm cells using micromanipulation equipment. Approximately 5 cells are extracted while avoiding the inner cell mass. These cells will undergo whole-genome amplification and then be tested using the personalized designed probes. The following are some of the PGT-M case studies conducted at our hospital.
PGT-M Monogenic Autosomal Recessive Genetic Disease Case: Pompe Disease
Pompe Disease, also known as acid maltase deficiency or glycogen storage disorder II, is a rare autosomal recessive genetic disease. Patients with this condition experience progressive heart muscle weakness and skeletal muscle weakness due to mutations in the acid alpha-glucosidase (GAA) gene, which leads to enzyme deficiency.In this case, the first child of the couple was diagnosed with Pompe Disease. After both spouses underwent carrier screening for genetic diseases, it was found that they were both carriers of Pompe Disease. As a result, they sought PGT-M testing for embryo screening. Based on the testing results during the procedure, they ended up with one affected embryo, two carrier embryos, and one unaffected embryo.
PGT-M Monogenic Autosomal Dominant Genetic Disease Case: Spinocerebellar Ataxia Type 3 (SCA3)
Spinocerebellar ataxia, also known as "SCA," is an inherited genetic disease caused by mutations in the deoxyribonucleic acid (DNA).In this case, the couple had a significant family history of Spinocerebellar ataxia. Therefore, they sought IVF treatment and PGT-M testing at Lee Women's Hospital. The testing results during the procedure revealed two embryos without the mutated gene. After implanting one of these embryos, the patient became pregnant, and subsequent amniocentesis results were normal, leading to a successful delivery.
PGT-M X-linked Recessive Monogenic Disease Case: Hemophilia B
Hemophilia B is a hemorrhagic disease caused by congenital deficiency of blood clotting factors F9. Since it is sex-linked, males with the carrier gene are affected, while females are usually carriers without symptoms.The patient had a family history of Hemophilia B, with the mother being a carrier and the brother being affected by Hemophilia B. After confirming that the patient is a carrier of Hemophilia B, they chose to undergo PGT-M testing. In the first attempt of IVF treatment, they obtained an unaffected embryo and implanted it. The patient successfully became pregnant, and the subsequent amniocentesis results were normal, resulting in a successful delivery.




